Carnot Cycle Which of These Following Statements Is True
Check All That Apply Being a real cycle all processes in a Carnot cycle are irreversible. A One complete Carnot cycle generates a vanishing change of entropy.

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics
A Carnot cycle is a theoretical ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s.
. No refrigerator using reversed Carnot cycle has been constructed because a it is less efficient b it is uneconomical c isentropic portions of cycle require low speeds where as isothermal portions require high speeds d isentropic portions of cycle require high speeds where as isothermal portions require low. There is an entropy increase during the isothermal expansion. Which of the following is the primary reason the efficiency is lower.
D The Carnot cycle is the basis for climate change. The work of the cycle amount of reheat respectively are S Thermodynamics Engineering. AIt is a cycle in which the efficiency is independent of the working substance.
Consider the following statements regarding Otto cycle. For a Carnot Cycle which of the following statements are true. The working fluid is air.
In a Carnot cycle the system executing the cycle undergoes a series of four internally reversible processes. C Sampling increases the amount of elements in a data stream. Of these two engines R is reversible and I is irreversible.
A refrigerator is basically a heat pump used to pump the heat absorbed from the material at lower temperature inside the refrigerator into surrounding at higher temperature The coefficient of performance of a refrigerator is defined as ratio of heat removed Q 2. C The Carnot cycle can be analyzed to prove that entropy is a state function. The Calvin cycle named after the tribute to Melvin Calvin who discovered it is the main route of carbon dioxide fixation.
A reversible cycle has following processes. B The total internal energy change around a complete Carnot cycle is negative meaning it does work or expels heat. Carnot Cycle Processes.
All of the above. A Sampling reduces the amount of data fed to a subsequent data mining algorithm. The steam is further expnaded from a state where enthalpy is 2950 kJkg to enthalpy of 2525 kJkg latent heat of 2201 kJkg.
The pump is not reversible. In gives one power stroke in the four strokes ie. Carnot heat engine refrigerator or heat pump that operates on a Carnot cycle Carnot principle principle governing the efficiency or performance of a heat device operating on a Carnot cycle.
In a the process is reversible isothermal gas expansion. The condenser is not isothermal. B Sampling reduces the diversity of the data stream.
In two revolutions of the crankshaft. A heat pump is another device that transfers heat from a low-temperature medium to high-temperature medium. The practical way of increasing its efficiency is to increase the compressionratio.
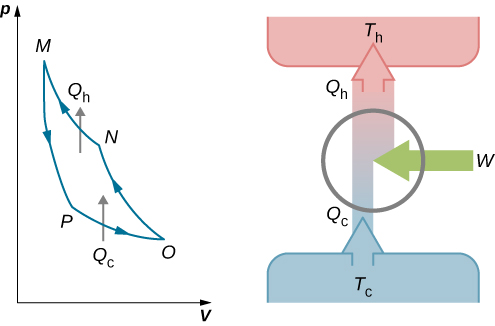
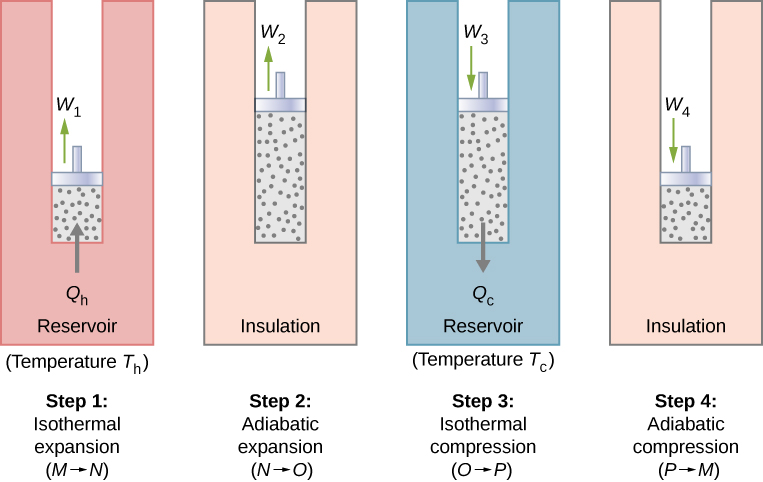
Isentropic compression The gas is compressed adiabatically from state 1 to state 2 where the temperature is T H. Its efficiency can be improved by using a working fluid of higher value ofratio of specific heats. On the Carnot graph the area inside the cyle is equal to the work out for a.
Possible but with lot of sophistications. C O P H P D e s i r e d O u t p u t R e q u i r e d I n p u t Q 1 W Q 1 Q 1 Q 2 T 1 T 1 T 2 T H T H. In this process the amount of heat absorbed by the ideal gas is qin from the heat source at a temperature of Th.
All the heat engines are based on Carnot cycle. The ideal Rankine cycle has lower efficiency than a Carnot cycle operating between the same two temperatures. A Carnot power cycle consists of 2 isentropic processes and 2 isothermal processes.
As such three strokes are idle strokes. A heat pump is a heat engine working in reverse direction. The condenser over cools the liquid vapor mix.
In b the process is reversible adiabatic gas expansion. C It is a cycle modeled by a closed system. E Sampling aims to keep statistical properties of the data intact.
Which of the following isare true about the Carnot cycle. It is not a reversible cycle. The boiler is not isothermal.
All of the following statements about the Carnot cycle are True EXCEPT. A reversible cycle is an ideal hypothetical cycle in which all processes are reversible. Such a proposition is.
The objective of a heat pump is to maintain a heated space at high temperature by supplying heat desired output to it. The thermal efficiency of a Rankine cycle can be made to be equal to that of a Carnot power cycle operating between the same cold and hot. Suppose I is more efficient than R.
It is used as the alternate standard of comparison of all heat engines. B It is a cycle in which the net heat is the same as net work. In a reheat Rankine cycle steam with enthalpy of 3300 kJkg is expanded inhp turbine to a state where enthalpy is 2650 kJkg.
2 marks All carnot cycles which operate between two finite temperatures must reject some heat to the lower. A true b false Answer. Two of the processes are isothermal.
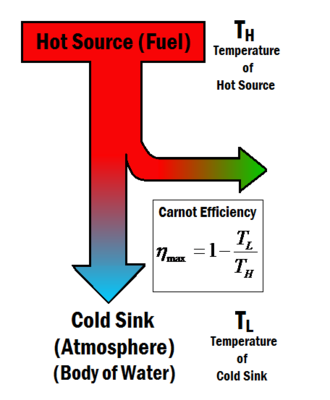
It provides an upper limit on the efficiency that any classical thermodynamic engine can achieve during the conversion of heat into work or conversely the efficiency of a refrigeration system in creating a. DIt is a cycle modeled by four isentropic processes. Carnot cycle is a reversible cycle.
Textbook chapter 10 Irreversibilities are present in the Carnot Cycle. This cycle does not release oxygen as a byproduct. Carnots Cycle Proof First Part To prove the first part of the theorem we consider two engines R and I working between the temperatures T 1 and T 2 where T 1 T 2 Fig.
This cycle is formed by reactions that occur in the chloroplast stroma and is one of the stages of photosynthesis as it occurs during the dark phase of photosynthesis. Which of the following statements are true regarding the Carnot Cycle. It provides concept of maximising work output between the two temperature limits.
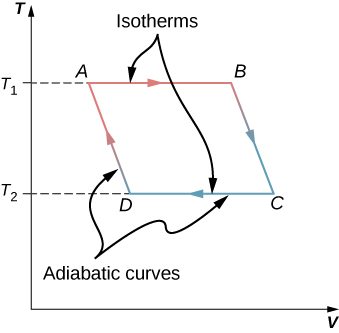
Following are the four processes of the Carnot cycle. 1 Which of the following statements are true of Carnot Cycles. I and II only I and III only All three statements are correct.
All carnot cycles which operate between two finite temperatures must have energy wastedlost due to the rejection of heat to the lower temperature reservoir. A 4 isothermal processes b 4 adiabatic processes c 2 isothermal and 2 adiabatic processes d none of the mentioned Answer. An actual engine is to be designed having same efficiency as the Carnot cycle.
All four of the processes are adiabatic. There is no entropy change during the adiabatic processes. No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between those same reservoirs.
D Sampling increases the amount of data fed to a data mining algorithm. The net entropy change for one cycle is zero. A Carnot Cycle consists of reversible processes only The Carnot Cycle is not a realistic model for real vapor power cycles The Carnot Cycle cannot be approximated using actual devices.
Share this link with a friend. Which is the incorrect statement about Carnot cycle. Check all that apply.
Any reversible heat device working between two reservoirs must have the same efficiency or performance coefficient greater than that of an irreversible heat device operating between the. Two isentropic processes reversible adiabatic alternated with two isothermal processes. The gas expands and does work on the surroundings.
What is true of the Carnot Cycle. In this type of engine one power stroke is obtained in two revolutions of the crankshaft. In this engine one power stroke is obtained in each revolution of the crankshaft.

Carnot Cycle P V Diagram And T S Diagram
Refrigeration Cycles Mech Engineering Thermodynamics Ucl Wiki

Efficiency Of A Carnot Engine Video Khan Academy
What Modifications Can Be Made On A Carnot Cycle To Make It Practical Quora

Carnot Cycle An Overview Sciencedirect Topics

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics

Carnot Cycle An Overview Sciencedirect Topics
The Carnot Cycle University Physics Volume 2
Carnot Efficiency Energy Education

Carnot Cycle An Overview Sciencedirect Topics

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics

The Carnot Cycle University Physics Volume 2

Carnot Cycle An Overview Sciencedirect Topics
The Carnot Cycle University Physics Volume 2
Entropy Free Full Text The Carnot Cycle Reversibility And Entropy Html

Survival Gear Buyer Beware Caveat Emptor Stirling Engine Survival Gear Survival

Chapter 5 The Second Law Of Thermodynamics Updated 7 5 2014

The P V Indicator Diagram Of A Carnot Cycle 1 2 3 4 Where T C T H Download Scientific Diagram

Comments
Post a Comment